electronic configuration of iodine|writing electron configurations for ions : Manila The total number of electrons in iodine is fifty-three. These electrons are arranged according to specific rules in different orbitals. . Tingnan ang higit pa Renaissance Wind Creek Aruba Resort 109 of 109 - Renaissance Wind Creek Aruba Resort RENAISSANCE® WIND CREEK ARUBA RESORT. Overview Gallery Accommodations Restaurants & Bars Experiences Meetings & Weddings Renaissance Island. Follow Renaissance Wind Creek Aruba Resort. Facebook Twitter Instagram. .
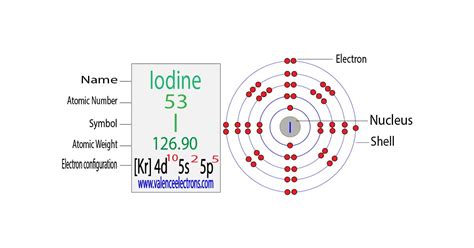
electronic configuration of iodine,Atoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground state electron configuration of iodine is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5. In the iodine ground-state electron configuration, the last electrons of the 5p orbital are located in the . Tingnan ang higit pa
The total number of electrons in iodine is fifty-three. These electrons are arranged according to specific rules in different orbitals. . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit paAfter arranging the electrons, it is seen that the last shell of the iodine atom has seven electrons. Therefore, the valence electronsof iodine are seven. The elements that have 5, 6, or 7 electrons in the last shell receive the electrons in the last shell . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit pa Mar 23, 2023 Electronic configuration of the Iodine atom. Valence electrons. Orbital diagram.electronic configuration of iodine writing electron configurations for ions Iodine Electron Configuration: Iodine is a chemical element that has a symbol I. The atomic number of Iodine is 53. It is the heaviest of the stable halogens. It exists as a purple-black, lustrous, non-metallic .Iodine is the fourth halogen, being a member of group 17 in the periodic table, below fluorine, chlorine, and bromine; it is the heaviest stable member of its group. (The fifth and sixth halogens, the radioactive astatine and tennessine, are not well-studied due to their expense and inaccessibility in large quantities, but appear to show various unusual properties for the group due to relativistic effects.) .
Learn how to write the electronic configuration of Iodine (I) and how to distribute electrons in different shells and subshells. Find out the valence electrons and .
Iodine is a chemical element of the periodic table with chemical symbol I and atomic number 53 with an atomic weight of 126.904 u and is classed as nonmetal and is part of . Let us discuss the energy levels that enclose an atom. The electronic configuration of [I] is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4d10 5s2 5p5, with atomic number .Full electron configuration of iodine: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5. tellurium ← iodine → xenon. Iodine, complete electron configuration. IODINE. HALOGENS ELEMENT. Iodine was discovered by Bernard Courtois (FR) in 1811. The origin of the name comes from the Greek word iodes meaning violet. It .
Configuration. σ g π u 4 π g 3 σ u 2. 10 . of the D state excited by the 1830 atomic line of iodine. The system further includes the diffuse emission bands in the region 2500 - 5000 with a . Schwarz, W.H.E., Inner electron excitation of iodine in the gaseous and solid phase, J. Chem. Phys., 1973, 58, 2230. Venkateswarlu, 1970 .Configurazione elettronica dello iodio. La configurazione elettronica dello iodio è 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s²4d¹⁰ 5p⁵. Lo iodio, detto anche iodio è definito come l'elemento chimico che appartiene alla tavola periodica. Si trova nel gruppo 17, più precisamente negli alogeni, il suo numero atomico è 53 ed è .

Iodine is a chemical element of the periodic table with chemical symbol I and atomic number 53 with an atomic weight of 126.904 u and is classed as a nonmetal. . Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 10: 4s 2: 4p 6: 4d 10: 5s 2: 5p 5: Electrons per shell: 2, 8, 18, 18, 7: Valence electrons : 7: Valency electrons : 1,3 .
A step-by-step description of how to write the electron configuration for Iodine (I). In order to write the I electron configuration we first need to know t.
Iodine is a 5-period element with a total of 53 electrons, filled in the ascending series of energy levels 1s, 2s, 2p, and so on. The s, p, d and f orbitals can hold a maximum of 2, 6, 10 and 14 electrons respectively. The configuration of I is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4d10 5s2 after filling 48 electrons entirely with respective subshells.electronic configuration of iodineThe Electron configuration of iodine is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s²4d¹⁰ 5p⁵. Iodine, also called iodine is defined as the chemical element that belongs to the periodic table. It is located in group 17, more precisely in the halogens, its atomic number is 53 and it is represented by the symbol I. This element can be .
Iodine: Iodine is a p-block element having an atomic number 53 and an atomic symbol I. It belongs to the Halogen family i.e. Group-17. It is a non-metallic solid with a semi-lustrous appearance. Electronic configuration of Iodine: The electronic configuration of a neutral atom of Iodine is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 .The radioactive isotope iodine-131 is sometimes used to treat cancerous thyroid glands. Iodine is an essential element for humans, who need a daily intake of about 0.1 milligrams of iodide. Our bodies contain up to 20 milligrams, mainly in the thyroid gland. This gland helps to regulate growth and body temperature.

The electron configuration in the outer shell is \(ns^2np^5\). As the atomic number increases, the reactivity of the halogens decreases. Fluorine and chlorine exist as gases at room temperature, while bromine is a liquid, and iodine is a solid. Review. Pick two elements that are halogens. For each, write the name, chemical symbol, and atomic .
La configuración electrónica del yodo es 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s²4d¹⁰ 5p⁵. El yodo, también llamado yodo, se define como el elemento químico que pertenece a la tabla periódica. Se encuentra en el grupo 17, más precisamente en los halógenos, su número atómico es 53 y se representa con el símbolo I. Este . Iodine -. I: properties of free atoms. Iodine atoms have 53 electrons and the shell structure is 2.8.18.18.7. The ground state electron configuration of ground state gaseous neutral iodine is [ Kr ]. 4d10. . Electron Configuration and Oxidation States of Iodine. Electron configuration of Iodine is [Kr] 4d10 5s2 5p5. Possible oxidation states are +1,5,7/-1. Electron Configuration. The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical .The ground state electron configuration of ground state gaseous neutral iodine is [Kr].4d 10.5s 2.5p 5 and the term symbol is 2 P 3/2. Schematic electronic configuration of iodine. The Kossel shell structure of iodine. Atomic spectrum . A representation of the atomic spectrum of iodine.碘的电子构型. 碘的原子序数是 53,这意味着它有 53 个价电子。. 原子的每个轨道和能量子水平都通过雨法完成,直到达到该元素中的所有 53 个电子。. 因此碘的完整电子构型如下: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s²4d¹⁰ 5p⁵. 简写或简化的电子构型如下 .
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ). In this video we will write the electron configuration for I- the Iodide ion. We’ll also look at why Iodine forms a 1- ion and how the electron configuration.writing electron configurations for ionsElectronic configuration: [Kr] 4d 10 5s 2 5p 5: Formal oxidation number:-1 +1 +5 +7: Electronegativities: 2.66: Atomic radius / pm: 133.1: Relative atomic mass: 126.904 47(3) Iodine was discovered by Bernard Courtois (FR) in 1811. The origin of the name comes from the Greek word iodes meaning violet. It is a shiny, black, non-metallic solid .
As mentioned above, the electron configuration of iodine is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 5. Hence, draw the blank orbital diagram of iodine up to 5p subshell as follows: Blank orbital diagram of iodine. In the above orbital diagram, the box represents an orbital. Each orbital has a capacity of two electrons.
electronic configuration of iodine|writing electron configurations for ions
PH0 · writing electron configurations for ions
PH1 · valence electrons of iodine
PH2 · unabbreviated electron configuration iodine
PH3 · iodine periodic table
PH4 · iodine electronic structure
PH5 · iodine condensed electron configuration
PH6 · iodine abbreviation electron configuration
PH7 · ground state electron configuration iodine
PH8 · Iba pa